
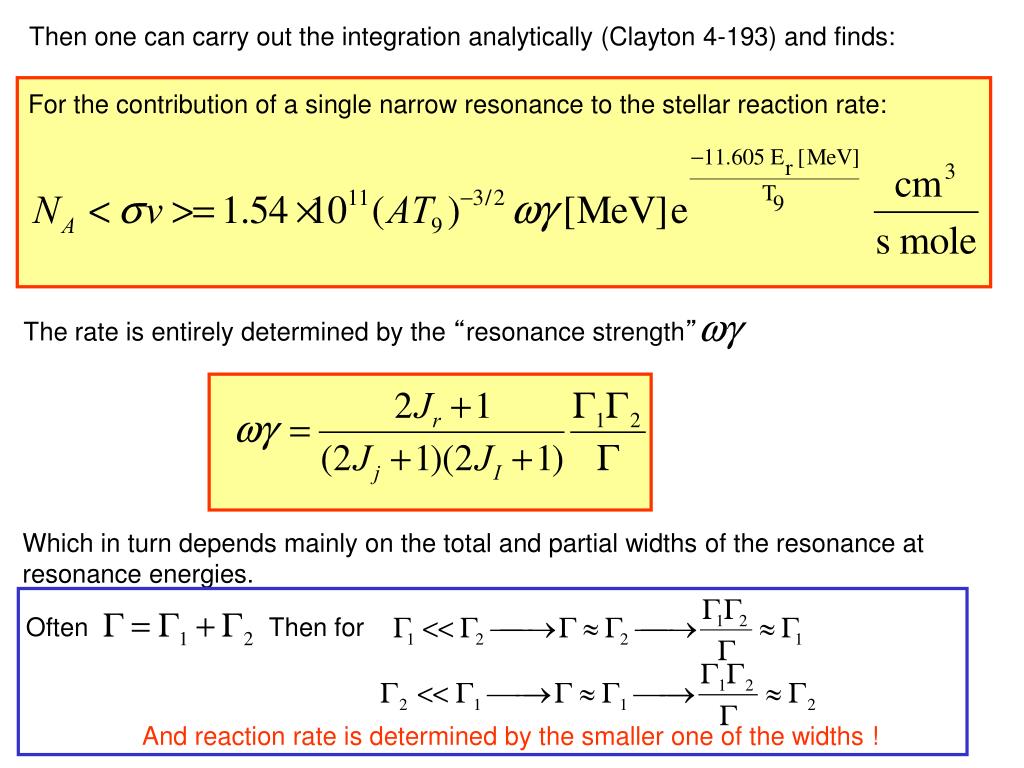
Nuclear Physics: Beta Decay Example: A thorium nucleus spontaneously decays, producing a protactinium nucleus and an beta particle. Nuclear Physics: Alpha Decay Example: A uranium nucleus spontaneously decays, producing a thorium nucleus and an alpha particle. Nuclear Physics: Radioactive Decay a particle: most massive, least “penetrative” b particle: “non-superlative” mass and penetration g particle: least massive (massless), most “penetrative” p+ no no Alpha Particle (a) Two protons and two neutrons (helium nucleus) p+ Beta Particle (b) A high energy electron Gamma (g) Particle (Ray) A high energy photon Nuclear Physics: Radioactive Decay There are three different types of “particles” that can be given off during radioactive decay. Instead of an equation with an equal sign, a reaction is shown with an arrow.

Nuclear Reactions During nuclear reactions, both mass and charge numbers must be conserved.
#Nuclear physics lectures ppt plus#
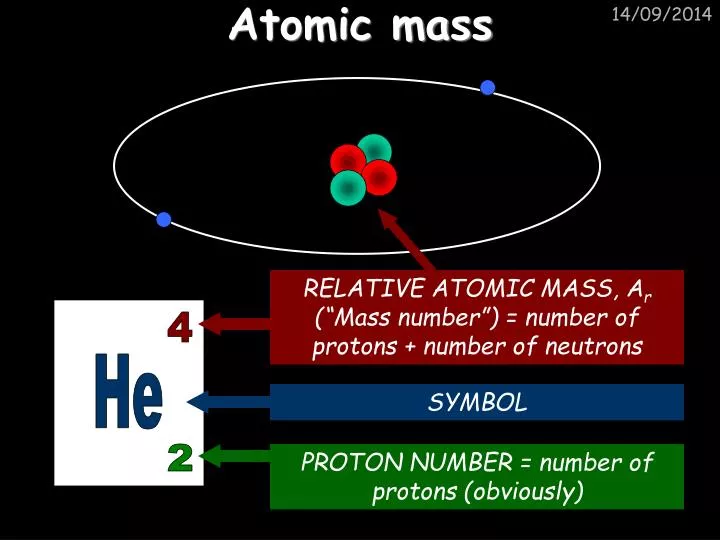
Mass Number: Number of protons plus neutrons Element Symbol Atomic Number = number of protons (or # of positive charges) Electron Neutron Proton Nuclear Physics: Notation This notation can also be used for single particles… Nucleons - the two constituent particles of a nucleus (protons and neutrons).Isotopes - atoms of the same element with different numbers of neutrons.


 0 kommentar(er)
0 kommentar(er)
